Acid Base Titration Lab Report Answers
You will be given a checklist of rules to read. Acidbase Solves for pH of strong and weak acids bufferspH during titrations and more.
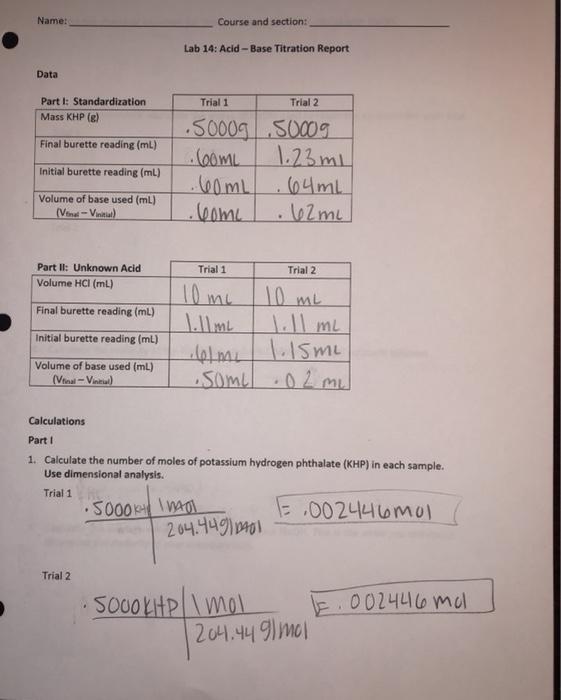
Solved Name Course And Section Lab 14 Acid Base Titration Chegg Com
By observing the titration of a strong acid and strong base and a strong base and weak acid.

. Titration Analysis of Aspirin Tablets. A metalloid is a type of chemical element which has a preponderance of properties in between or that are a mixture of those of metals and nonmetalsThere is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity the term remains in use in the literature of chemistry.
Experiment 1011Part 1 Acid Base Titration. Back to Home Page. Biometric technology ensures that.
Kb of aniline is 383 x 10- 3241 O 10759 6543 None of. Sample Preparation Procedure for Spectrochemical Determination of Total Recoverable Elements. The purpose of this experiment is to observe the titration of hydrochloric acid a strong acid with sodium hydroxide a strong base and acetic acid a weak acid with sodium hydroxide a strong base.
This experiment is designed to determine the molar concentration of acetic acid in a sample of vinegar by titrating it with a standard solution of NaOH. Solve the math fact fluency problem. Upon check-in Pearson VUE will collect your signature photographic image and palm vein image.
Methylamine CH3NH₂ has a base-dissociation constant of 438 x 10-4. Another palm vein image will be taken before you are seated in the exam room and if you leave the room during your exam. Acidity Acidity is a program that calculates the pH of an acid or base.
Also determine whether the aspirin is a strong or weak acid according to the Bronsted- Lowry and Lewis theories and deduce the formula of the acid- base. Preparation and standardization of -1 Sodium hydroxide 2 Sulphuric acid 3 Sodium thiosulfate 4 Potassium permanganate 5 Ceric ammonium sulphate. Assay of the following compounds along with Standardization of Titrant- 1 Ammonium chloride by acid base titration 2 Ferrous sulphate.
Number Method Title. Determine the percentage of aspirin acetylsalicylic acid present in two different commercial tablets by titrating the solution with a base. Introduction Vinegar is a common household item containing acetic acid as well as some other chemicals.
Trace Elements in Drinking Water by Axially Viewed Inductively Coupled Plasma-Atomic Emission Spectrometry. The six commonly recognised metalloids are. Limit Tests- 1 Chloride 2 Sulphate 3 Iron 4 Arsenic.
Adaptive and individualized Reflex is the most effective and fun system for mastering basic facts in addition subtraction multiplication and division for grades 2. This simple easy program helps calculate pH pOH OH- and H for acid and base problems. 6 Acid Base and Salt 136 143 61 The Role of Water in Showing Acidic and 149 Alkaline Properties 152 158 62 pH Value 162 63 Strength of Acids and Alkalis 167 64 Chemical Properties of Acids and Alkalis 174 65 Concentration of Aqueous Solution 178 66 Standard Solution 190 67 Neutralisation 197 68 Salts Crystals and Their Uses in Daily Life 216 69.
LOG IN 0 ITEMS. Calculate the pH of 0800 M methylammonium chloride CH3NH₂C 5369 8631 9765 None of these 18. You must report to the testing center 30 minutes prior to your scheduled time.
CH3COOHaq NaOHaq - CH3COONaaq H2Ol By adding the sodium hydroxide which. It also helps you show your work. Calculate the pH of a buffer that contains 0270 M aniline C6H5NH₂ and 0180 M anilinium chloride C6H5NH3CI.
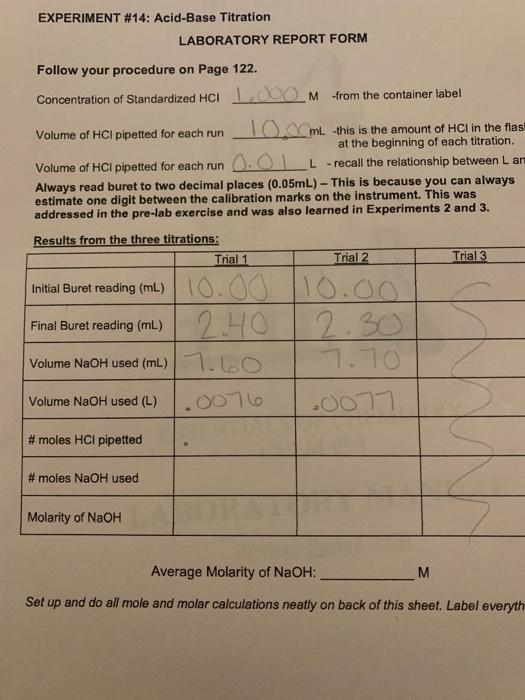
Solved Experiment 14 Acid Base Titration Laboratory Report Chegg Com
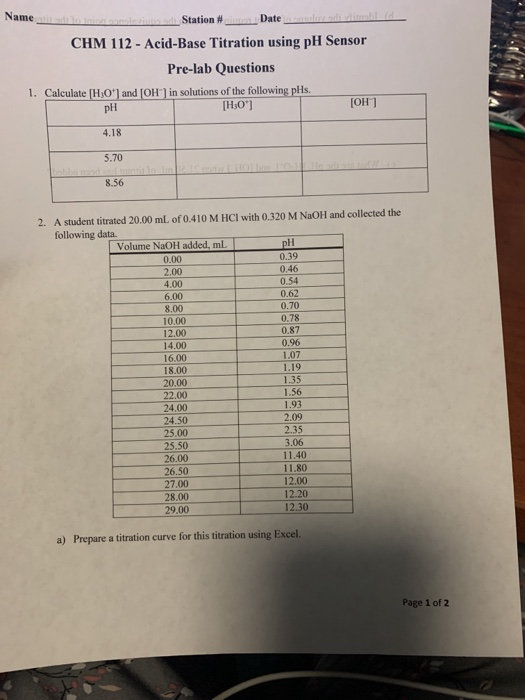
Solved Wame S Tation Date Chm 112 Acid Base Titration Chegg Com

Titration Practical And Calculation Naoh And Hcl Teaching Chemistry Chemistry Lessons Chemistry Practical

Solved Experiment 7 Analysis Of Unknown Acids By Acid Base Chegg Com
No comments for "Acid Base Titration Lab Report Answers"
Post a Comment